Challenges Of NIAS Towards Food Packaging Industry – A Critical Review
Non-intentionally added substances (NIAS) are chemicals that have not been added for a technical reason during the total production cycle of food packaging manufacturing process. The control of chemical migration of NIAS and their identification in the food packaging are quite complex, and a big threat to the food packaging Industry. In this article, an attempt has been made to provide a comprehensive review of available literature on this important concept.
What Are NIAS (Non-Intentionally Added Substances)?
The objective of food contact materials (FCM) or food contact article (FCA) is to package and safeguard food during transportation and storage, to increase shelf life or for branding purposes. It includes all materials and articles intended to come into contact with food. (FCM) can release substances into food and beverages to which the consumer will be exposed to through their diet. These substances can be intentionally added substances (IAS) or non-intentionally added substances (NIAS). Non-intentionally added substances (NIAS) are chemicals that have not been added for a technical reason during the production process. The presence of, possibly toxic, NIAS is often not known by the manufacturer itself.
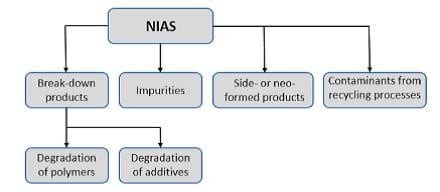
Figure 1: Classification Of NIAS
Classification of NIAS
The primary sources from which NIAS may formed from packaging materials can be classified into reaction by-products, oligomers, break-down or degradation products, impurities from raw materials, side products or neo-formed compounds, and contaminants picked up during the production or recycling process. Degradation products can be further divided into degradation of polymers, and degradation of additives (Fig. 1).
MAJOR REGULATIONS ON NIAS
EUROPE
In Europe, the concept of NIAS is not new, but has raised awareness since they were specifically mentioned in Article 19 of Regulation EU 10/2011 (European Commission, 2011). This Regulation states that: “NIAS are permitted in final plastic articles, but should be assessed by the manufacturer in accordance with international recognised scientific principles on risk assessment”. On January 28, 2016, the European Food Safety Authority (EFSA) Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids concluded that the required toxicity data for substances in FCM’s (IAS and NIAS) should be related to the expected human exposure and proposed three threshold levels of human exposure as triggers for requiring additional toxicity data.
However, a quantitative analysis of NIAS is not always possible since reference standards are not available. Particular attention has been given to NIAS with a molecular weight (MW) below 1,000 Da, as it is generally recognised that components with a molecular mass above 1,000 Da are not absorbed by the gastrointestinal tract and therefore should not represent a risk to consumer health, with the exception of fluorocarbons for which this threshold is 1500 Da since at the same molecular weight, fluorocarbons tend to have a smaller molecular volume.
UNITED STATES
As per U.S. Food and Drug Administration (FDA), the term NIAS is not used in a legal context. However, there are provisions concerning impurities (21 CFR 174.5) and substances originating from direct contact between a food contact substance (FCS) and the food (21 CFR 170.3(i)). The U.S. Food and Drug Administration (FDA) recommends including information on the major impurities (e.g., residual starting materials, by-products, degradations products) when submitting a food contact notification or food additive petition for an FCS.
CHINA
A definition for NIAS is provided in Standard GB 4806.1. Manufacturers shall perform a risk assessment to assess the safety of NIAS, but explicit approvals are not foreseen.
INDIA
As per available Indian regulations, the concept of NIAS is not directly present in any of packaging regulations or standards. The Indian Standard IS 15495 clearly states that it is the responsibility of the printer and the converter is to ensure that the food packages are manufactured and stored in a manner by which any preventable transfer of material from the ink or coating to the food contents is avoided, even if such transfer is unobjectionable on the grounds of health, odour and flavour. Similarly, the IS 16021: 2012, Good Manufacturing Practices (GMP) —Requirements for Organizations in the Food Processing Sector, and the IS 16020: 2012 Food Safety Management— Requirements for Good Hygiene Practices defines ‘Contaminant’ as “any biological, chemical agent (including allergens), foreign matter, or other substances not intentionally added to food, which may compromise food safety or suitability.”
NIAS FORMATION AND ANALYTICAL METHODOLOGY FOR IDENTIFICATION
The identification and quantification of NIAS is quite difficult as in most cases the composition of the formulations used in the manufacture of FCM is not fully known or because of the complexity of the packaging (e.g., multilayer materials), as well as considerations relating to chemical analysis. Advanced analytical techniques, namely, gas chromatography (GC)–mass spectrometry (MS) for volatile and semi-volatile compounds, and liquid chromatography (LC) coupled to MS for non-volatile compounds have been applied to address the challenge of identifying NIAS. Figure 2 shows a scheme of an analytical procedure to evaluate NIAS.
NIAS formation through Degradation processes
One of the most frequent pathways to NIAS formation is degradation processes. Degradation can take place in the polymer itself and also in the additives used for improving its physicochemical characteristics. The main sources of degradation include exposure of the polymer to the high temperatures or high irradiation energies that can occur during polymer manufacturing, exposure to microwaves or irradiation processes, for example when gamma or beta irradiation is applied to packaged food for sterilization purposes, or exposure to thermo-mechanical processes. During these processes, new molecules with a lower molecular weight can be formed and these molecules will have higher diffusion coefficients and therefore a higher migration potential. Although the irradiation of packaged food is not yet a widespread form of sterilization, in some countries it is applied to specific foodstuffs. There is a maximum limit when gamma or beta irradiation are used, usually 10 kg, but in any case the stability of the packaging materials should be confirmed.
NIAS formation through additives degradation
Some additives such as antioxidants or light stabilizers added to the polymer for improving their properties can also be degraded. As a result, new potential migrants will be present in the packaging material. Other degradation products that have been studied owing to their toxicity are the alkylphenols, nonylphenol (NP) and octylphenol (OP), which are known to be endocrine disrupters. NP and OP can be generated by the oxidation of tris(nonylphenyl)phosphite (TNPP), used as an antioxidant in polymeric materials. Not only the additives in the polymer but also those additives added to adhesives, coatings or inks used in food packaging manufacture can suffer degradation processes and provide NIAS to the material and their subsequent migration.
NIAS formation through impurities
Another frequent reason for finding NIAS in migration from food packaging is the presence of impurities coming from the raw materials or additives used during polymer manufacturing. These impurities are not described in the information datasheets of the starting substances and therefore it is not possible to know their identity in advance. In some recently published articles focused on the development of new active packaging, impurities from active agents used to provide active properties to the packaging have been identified in the specific migration analysis, either in food simulants or in food. Impurities from inks and adhesive additives used in food packaging have also been found in migration.
NIAS formation through Neoformed compounds
One of the reaction products that have been found in migration and that have adverse effects on consumer health are the primary aromatic amines (PAAs), suspected to be carcinogenic compounds. They appear as neoformed compounds from polyurethane (PU) adhesives, commonly used for preparing food contact multilayer laminates. PU adhesives are formed by the polymerization of polyols and diisocyanate monomers. If the adhesive has not been properly cured or if the ingredients have been mixed wrongly, the polymerization reaction will not be efficient enough and the remaining unpolymerized aromatic isocyanates will come into contact with water, producing primary aromatic amines (PAAs). It was observed that when azo dyes were used in printing inks, the chromophoric-azo groups could, under certain conditions, be reduced and form aromatic primary amines, with a known toxic effect. Another food packaging additive that has been found to react with chlorine is epoxidized soybean oil (ESBO). ESBO is one of the most extensively used additives for food-contact PVC gaskets. When PVC is subjected to high temperatures, HCl is released and a further reaction of this HCl with an epoxy group of ESBO takes place, forming chlorohydrins.
NIAS formation through Contaminants
When recycled materials are used for food packaging, the possible migration of NIAS coming from contamination of the packaging has special relevance. These contaminants may be chemical compounds coming from the previously packaged food, but also substances resulting from the misuse of the packaging by the consumer before discarding it. The presence of intrinsic contaminants from the recycling process, such as chemical additives and their degradation products also has to be considered. Most of the studies about migration from recycled materials have been performed with PET. Paper and board polyolefins have also been studied. Polyolefins and mainly LDPE are considered the worst-case migration materials as the diffusion coefficients of non-polar migrants in LDPE are very high, which means that small and medium size molecules are quickly transferred to the food in contact with them.
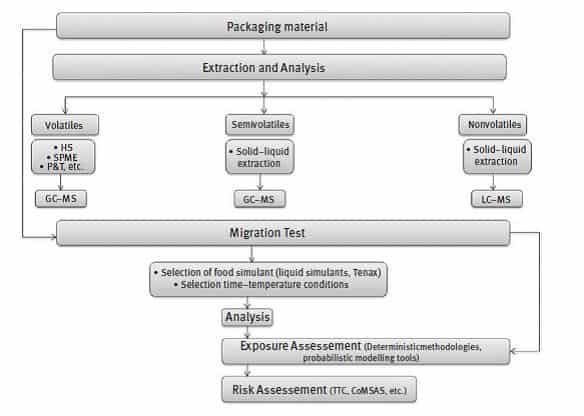
Figure 2: RISK ASSESSMENT OF NON-INTENTIONALLY ADDED SUBSTANCES
Risk Assessment of Non-Intentionally Added Substances
To evaluate the safety of FCM, it is necessary to know the migration of potential compounds from the packaging, which includes substances added intentionally during the manufacture of the materials and NIAS. How to approach the risk assessment of NIAS present in FCM is still under discussion. Different methodologies have been used for the risk assessment of FCM and the Threshold of Toxicological Concern (TTC) concept has been applied for this purpose. This approach is a screening tool that is used when the toxicity of a substance is unknown or the information is very limited. To apply this tool, it is necessary to know the chemical structure of the molecule and data regarding human exposure. It employs the Cramer decision tree that classifies substances according to their molecular structure into three classes, namely, Class I, II and III for substances with low, intermediate and high toxicity, respectively. The threshold for Cramer Class I, II and III are 1,800, 540 and 90 μg/person/day, respectively. The CoMSAS is another interesting methodology developed by Koster et al. In this approach, an exposure threshold of 90 μg/day is used, which corresponds to the threshold defined for Cramer Class III, whereas the conventional limit of detection of 10 μg/kg is used for migrants from FCM and substances over this value have to be identified. One benefit of this approach, from an analytical point of view, is that the identification can be simplified. Nevertheless, this method supposes that all the substances present a similar response with different detectors. In the near future, new tools will be explored for the risk assessment of unknown substances.
CONCLUSION
Analysis of NIAS was found to be very challenging since their presence and identity is often not known. The major sources of compounds found in packaging materials are components from printing inks, adhesives sizing agents, surface coatings, impurities in the raw materials and from the manufacturing process. Its success will depend on several factors such as the compound properties, their concentration in the sample, the availability of pure standards and the resolution achieved in the separation steps. Complex mixtures with co-eluting compounds or compounds with many possible isomers with similar physicochemical properties will significantly reduce the possibilities of an unequivocal analytical confirmation. Sample treatment procedures, separation techniques and concentration steps will be required in order to remove as much interference as possible and increase the concentration of target compounds. Software tools and database information together with high resolution MS are also essential for successful identification. Despite the complexity of NIAS identification, recent advances in analytical techniques have significantly contributed to facilitating this process. The development of powerful software tools for data treatment and the increase in chemical databases have also been essential for furthering this task. Continuous advances in this area are expected in the future.
Irrespective of the applied concept for risk assessment, communication and collaboration within the whole supply chain and regulatory bodies is essential to facilitate the prediction, identification, and quantification of NIAS.
PRESIDENT - R&D/INNOVATION at Yansefu Inks And Coatings
Mr. Neelakamal Mohapatra, M. Tech. in Chemical Technology from HBTI, Kanpur, India is a R&D, IPR and Product Safety Regulation personnel with 22 years of research experience primarily in the Printing and Packaging sector.
He focuses towards developing cutting edge products and processes for Flexible Packaging application. He has worked in various capacities with renowned printing ink, coating and adhesive manufacturing, and IPR organizations; and currently working with Yansefu Inks and Coatings Pvt. Ltd in the position of President-R&D/ Innovation.
His research work has been published in journals of national and international repute. Few of his innovations has also been patented. He has 30 publications and 16 patents registered on his name. His special interest in promoting product safety regulation (PSR) within printing inks and food packaging industries have been well acclaimed by various organizations.