Child-Resistant Packaging in Pharmaceuticals
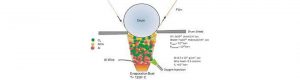
Child-Resistant packing in Pharmaceuticals is kept aside, as most of the drugs packed in HDPE containers with CR Closure. But for few years many Innovator drug products are available in Child-Resistant design (Blister Pack) and most of them are patented or difficult to make generic due to various reasons such as Cost, Availability of supplier and manufacturing feasibility. There Packaging professional has to take a call on new design development or to use a certified pack design. But when coming to the level of protection (F Level) required for a specific drug is became a challenge, because getting Toxicology data is difficult to decide “F” Value.