When it comes to packaging safety, every brand owner’s most significant concern is migration, regardless of the origin of the problem—e.g., substrate, adhesive, printing ink, overprint varnish, etc.
A particular focus lies on “bad surprise” chemical substances, coined as the next “Molecule of the Month,” with detrimental effects on human health, or taste and odor of the packed food and product recalls, contributing to court claims and severe brand damage.
Therefore, effective and intelligent risk mitigation measures have to be implemented at every stage of the packaging supply chain, along with open communication between the different stakeholders. This approach is responsible for leading to an increased level of transparency, ultimately resulting in food packaging that is compliant with all regulatory and brand owner demands, ensuring consumer safety.
Eventually, all these measures will create and sustain a high level of “perceived safety” on the consumer’s side, which is vital for all brands and products. Trust, which is built in years, can easily be destroyed in seconds.
Critical Communication
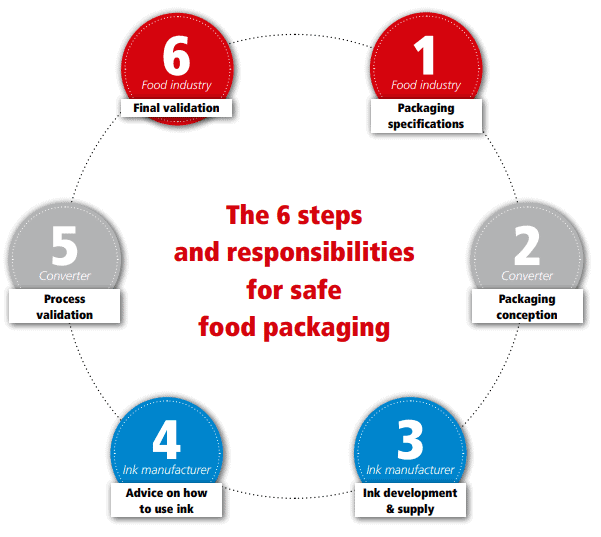
To produce compliant food packaging, communication has to take place at each interface of the supply chain.
The food producer issues packaging specifications that are transformed by the converter into a concrete packaging concept. Application-specific details have to be communicated to the ink supplier, who develops tailor-made solutions. If the inks are used in packaging for certain brand owners with their own specific requirements, such as Nestlé, the ink supplier has to be informed. Without this knowledge, the ink manufacturer may offer a non-compliant ink, which will result in non-compliant food packaging.
Once the correct inks are identified, the ink manufacturer has to pass on all necessary information to the converter, so the ink is able to be processed in the right way. This information has to cover technical aspects—e.g., suitable solvents for dilution, fastness and resistance properties, etc., as well as safety aspects—potential migrants, restrictions of use, worker safety aspects, etc.—to allow the converter to perform all necessary compliance work.
This compliance work includes the assessment of the migration level for potential migrants from the ink, based on the information provided by the ink manufacturer and of other suppliers, such as the substrate and adhesive manufacturers, considering the barrier properties of the substrates. Ideally, such an assessment results in a “Declaration of Compliance” for the subsequent stakeholder in the packaging supply chain.
The circle closes with final validation of the printed food packaging by the food producer who is placing the packaging into the market and is thus ultimately responsible for the overall compliance of the packaging.
…effective and intelligent risk mitigation measures have to be implemented at every stage of the packaging supply chain, along with open communication between the different stakeholders.
For special applications, like packaging for microwave, boil-in-the-bag and ovenable, or processing steps—retorting and sterilization—particular considerations may apply. Food packaging for especially sensitive consumer groups (say, babies) has to be evaluated even more carefully.
Compliance Tools
To manage the complex requirements for safe food packaging inks, the ink manufacturer has to implement a whole set of efficient and interdependent “compliance tools,” ever mindful of brand owner requirements.
Choice of the right raw materials: When it comes to ink formulation, the first step is the proper choice of the raw materials for the intended application. A powerful tool for guaranteeing the highest safety levels is the application of several layers of exclusion criteria.
Fundamental risks, such as carcinogenicity, reprotoxicity and mutagenicity, must be excluded in general, regardless of the application. The same holds true for several hazardous chemical substances, such as specific heavy metals, chlorinated hydrocarbons or dioxins. Siegwerk USA Inc, for instance, is committed to apply the Exclusion Policy for Printing Inks (EuPIA) and related products globally with its underlying exclusion criteria for various hazards.
On top of these basic criteria, special requirements for raw materials intended for food packaging inks apply. The chemical composition of all raw materials should be known down to trace levels, along with all non-intentionally added substances (NIAS), including the regulatory status of all potential migrants for all relevant regions of the world, present in the printed ink. All this information is the pre-condition for a proper and responsible evaluation.
It has proven helpful to hand over detailed raw material specifications, depending on the nature of the raw material (pigment, resin, additive, solvent) to the raw material suppliers to raise their awareness for printing ink-specific safety requirements and to establish an efficient and sustainable cooperation.
Lastly, only raw materials that do not inadvertently affect the food, in terms of odor and taste, can be used for printing inks for food packaging.
Advice on ink formulation: Printing inks for food packaging have to be formulated with the objective to minimize potential migration from the printed packaging into the food. To achieve the aforementioned task, all know-how that is collected in step one (choice of the right raw materials) has to be considered in the ink formulation process. Detailed knowledge about all potential migrants and their regulatory status has to be factored in, ultimately resulting in a migration-optimized ink formulation. Additionally, application, brand owner specifics and regional restrictions may apply, which makes the task even more challenging.
Very close cooperation between the product safety and technical departments is vital, and the challenge lies primarily in “translating” the complex regulatory data into explicit and straightforward rules for the ink formulators.
Full transparency at the interface to the converter: It is the task of the converter to assess all potential risks for the food packaging being printed. As such, it is vital to the converter’s compliance work to know the potential migrants of all relevant packaging components that are being combined—substrates, adhesives, printing inks, overprint lacquers, primers, etc.—including their quantity and regulatory status—migration limits, etc.
From the perspective of a printing ink manufacturer, it has proven to be useful to establish a so-called “Statement of Composition” (SoC), which is a standardized document, providing this information in an easy to use, comprehensive structure.
In absence of any legal framework for printing inks for food packaging in the US and Canada, Siegwerk has decided to encourage its customers to use the migration limits, as established in Europe and Switzerland for their compliance work. These limits are derived from toxicological data and thus provide a reasonable basis for consumer safety considerations, independent from the mere regional regulatory aspect.
“Trust, which is built in years, can easily be destroyed in seconds.”
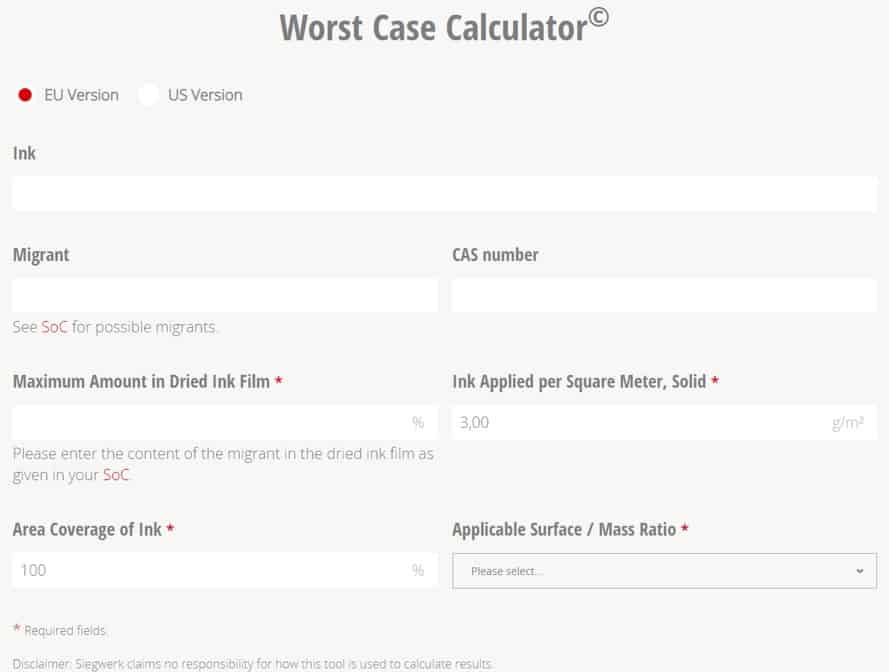
Within the SoC, each potential migrant is listed with its maximum amount in the dry ink film. These values are used as input parameters of a worst-case calculation (WCC), based on standard assumptions (100 percent migration of the substance, grammage, ink coverage, surface/mass ratio, etc.). The result of the WCC can then be compared to the migration limit of the substance (if applicable). If the result of the WCC is below the migration limit, that means the migration limit can never be reached or exceeded and the use of the substance can be considered as safe.
To make the WCC more realistic, the customer can use the real surface to mass ratio of the printed food packaging and perform a tailor-made calculation with the help of a WCC that is part of Siegwerk’s Ink Safety Portal and the data which are provided in the SoC.
Guidance and support: It is easy to get lost in the jungle of legal requirements, especially if it comes to global dimensions, including various regions of the world. The sheer handover of compliance documents, as described in the previous paragraph, is sometimes not enough, and there is a clear demand for expert guidance and support in the packaging supply chain when it comes to food packaging safety.
A printing ink manufacturer can make the life of many customers and brand owners considerably easier via offering hands-on support on ink selection, tailor-made food packaging safety workshops and training, as well as support on migration testing and joint migration risk assessment.
Proactive product safety work: Since the food packaging safety topic is quickly evolving and gaining pace all over the world, it is indispensable to stay “ahead of the game.”
Therefore, it is essential to care about the current compliance demands, as well as to deal with the topic proactively. This includes the monitoring of the continually evolving legal framework around the world, active participation in association work, support of the legislation process, lobbying activities, frequent exchange in expert forums, and strategic partnerships with customers and brand owners.
Packaging 360 is a comprehensive knowledge sharing ecosystem for the Indian packaging industry. Our services include an online content platform to deliver news, insights and case studies; organising conferences seminars and customised training; Providing Bespoke Project Consulting, Market Research and Intelligence.






