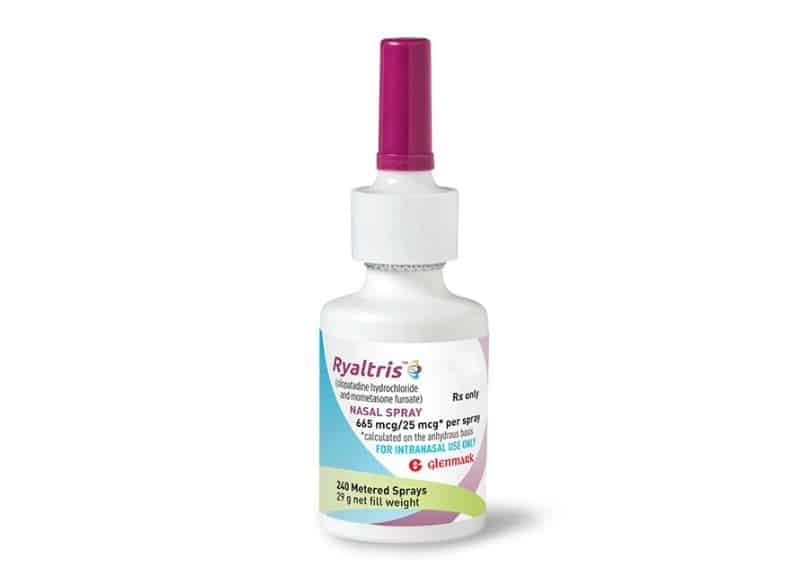
Aptar Pharma’s VP3 multidose device is the delivery system for Glenmark’s Ryaltris™ nasal spray, which recently received NDA approval by the US FDA for the treatment of symptoms of seasonal allergic rhinitis.
Aptar Pharma today announced that its VP3 multidose device is the delivery system for Glenmark’s Ryaltris™ nasal spray, which recently received New Drug Application (NDA) approval by the U.S. Food and Drug Administration (FDA) for the treatment of symptoms of seasonal allergic rhinitis in adults and pediatric patients 12 years of age and older.
Using Aptar Pharma’s innovative VP3 nasal spray with a custom-designed reduced evaporation cap, Ryaltris™ is a metered, fixed-dose, aqueous suspension prescription combination drug product nasal spray that combines an antihistamine (Olopatadine) with a steroid (Mometasone Furoate) for the treatment of symptoms of seasonal allergic rhinitis, including nasal and ocular symptoms.
The effort to bring Ryaltris™ Nasal Spray to market was supported by a Combination Product Documentation package from Aptar Pharma’s Services offering, a comprehensive portfolio of stage-specific development packages. Aptar Pharma’s dedicated Regulatory Affairs professionals and analytical scientists help customers proactively address regulatory needs to accelerate approval.
“Glenmark’s constant focus is on delivering the right treatment options to the patients, using effective technology. Adapting Aptar Pharma’s innovative VP3 multidose device as the delivery system for our novel Ryaltris™ nasal spray is yet another effort in this regard,” said Robert Crockart, Chief Commercial Officer, Glenmark Pharmaceuticals Ltd.
“This NDA approval by the U.S. FDA for a combination drug product using our VP3 multidose nasal pump further demonstrates Aptar Pharma’s ability to help our customers develop and launch novel treatments,” stated Gael Touya, President, Aptar Pharma. “Our nasal systems’ proven capabilities bring added value to our customers and further convenience for patients worldwide.”
The effort to bring Ryaltris™ Nasal Spray to market was supported by a Combination Product Documentation package from Aptar Pharma’s Services offering, a comprehensive portfolio of stage-specific development packages. Aptar Pharma’s dedicated Regulatory Affairs professionals and analytical scientists help customers proactively address regulatory needs to accelerate approval.
“Glenmark’s constant focus is on delivering the right treatment options to the patients, using effective technology. Adapting Aptar Pharma’s innovative VP3 multidose device as the delivery system for our novel Ryaltris™ nasal spray is yet another effort in this regard,” said Robert Crockart, Chief Commercial Officer, Glenmark Pharmaceuticals Ltd.
“This NDA approval by the U.S. FDA for a combination drug product using our VP3 multidose nasal pump further demonstrates Aptar Pharma’s ability to help our customers develop and launch novel treatments,” stated Gael Touya, President, Aptar Pharma. “Our nasal systems’ proven capabilities bring added value to our customers and further convenience for patients worldwide.”
Already approved and marketed in several countries across the world, Ryaltris™ will be marketed and distributed in the United States by Hikma Specialty U.S.A., Inc. as part of its exclusive licensing agreement with Glenmark Specialty S.A. (Switzerland)2
Packaging 360 is a comprehensive knowledge sharing ecosystem for the Indian packaging industry. Our services include an online content platform to deliver news, insights and case studies; organising conferences seminars and customised training; Providing Bespoke Project Consulting, Market Research and Intelligence.