To support its healthcare customers, Amcor is now launching in Europe a speciality multi-chamber pouch for drug-device combination products. Amcor’s Dual Chamber Pouch (DCP) has already been successfully tested and commercialised in the US market and won a 2021 Award from the Flexible Packaging Association.

Investing in the future of medical devices
The global market for medical device technologies is expected to grow by 5.6% over the next five years, and the global market for drug-device combination products (combination of a drug and a drug delivery device) is anticipated to rise to $186.7 billion by 2027, driven by a growing healthcare infrastructure and advances in drug delivery technologies.
At the same time that we witness the growth of combination devices, we see an increasing complexity of device constituents in drug-device combination products, and the need to protect the valuable device itself as well as the drug. Medical device regulation requires quality, safety and performance, with a greater focus on state-of-the-art requirements for all constituents of the device, including its packaging.
To support our healthcare customers, Amcor is launching in Europe a speciality multi-chamber pouch for drug-device combination products. Our Dual Chamber Pouch (DCP) has already been successfully tested and commercialised in the US market and won a 2021 Award from the Flexible Packaging Association for its technical innovation and material structure.
With 10 specialized sites across EMEA dedicated to healthcare packaging innovation, and an established R&D and consultancy team, Amcor’s Dual Chamber Pouch builds on years of experience in the medical and pharmaceutical industries to meet the complex requirements of medical device packaging.
Overcoming the challenges of packaging medical devices
Combination medical devices demand high performance packaging to ensure the sterility and shelf life of the product. Firstly, the packaging system needs to provide barrier against light, moisture and oxygen to maintain the stability and efficacy of the Active Pharmaceutical Ingredients (API). Secondly the pack needs to be suitable for sterilisation to deliver the device sterile at the point of use.
These two opposing but critical requirements of breathability for sterilisation and barrier pose a real challenge for packaging design. To overcome this, in standard packaging today, drug eluting stents (for example) are typically packaged in two separate pouches: the first pouch allows the ETO (ethylene oxide) sterilisation process, and a secondary outer foil pouch with a desiccant preserves drug stability.
Medical packaging that’s easy and intuitive to open
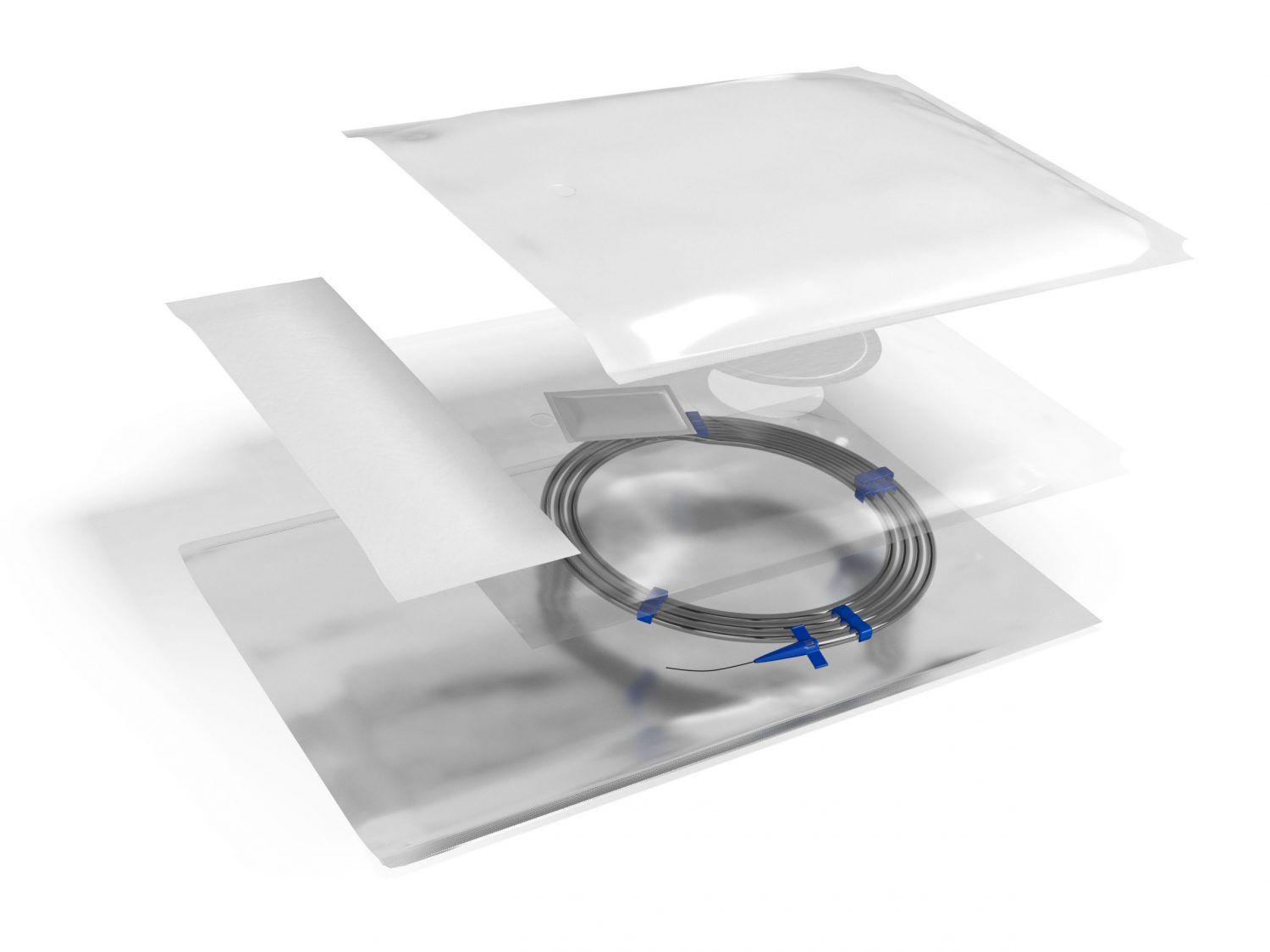
Amcor’s innovation is a safer and simpler design. It is one multi-compartment pouch with a breathable membrane separating the two chambers. The Amcor Dual Chamber Pouch uses a high strength foil laminate that protects from light, moisture and oxygen ingress to support shelf life and drug efficacy. One side is peelable and allows aseptic presentation and easy access to the device. The second non-peelable chamber contains the desiccant sachet or other scavenging technologies.

The internal breathable vent allows gas exchange for the desiccant to maintain a controlled environment within the pouch. The separate chambers eliminate any risk of the desiccant encountering the sterile device and sterile field. A porous header using DuPont™ Tyvek®* material is added to the pouch, which provides an easy method of ETO sterilisation, after which the pouch is sealed, the header removed, and the barrier pouch is ready for shipment.
Noemi Bertolino, Vice President R&D EMEA at Amcor said, “To protect a combination sterile device with a therapeutic drug agent, we knew there were a lot of requirements to fulfill. The first objective was to create a pack design that is easy and intuitive to open and can be aseptically presented. The integrity of the drug’s effective dosage also has to be maintained. To minimize the ingress of moisture vapor into the pouch, we created a vent towards the centre of the interior film, away from the seams. This solution maintains shelf life and minimises any risk of contamination. Our Dual Chamber Pouch complies with necessary medical and safety regulations, in an efficient, simplified design.”
Amcor’s Dual Chamber pouch will be available in Europe from our site in Sligo, Ireland, which is 100% focused on healthcare packaging. All production operates inside a certified cleanroom and uses the latest state-of-the-art inspection equipment for pouch making. Individual barcodes can be assigned to each pouch, providing full traceability, ensuring the highest standards of safety, quality and service are met for our customers.
By continuing to invest in all aspects of the packaging process – material, machines, research and development – Amcor can help healthcare providers to identify and develop solutions to existing challenges.
Packaging 360 is a comprehensive knowledge sharing ecosystem for the Indian packaging industry. Our services include an online content platform to deliver news, insights and case studies; organising conferences seminars and customised training; Providing Bespoke Project Consulting, Market Research and Intelligence.






